Pharmaceutical Strategy of The Norwegian Hospital Procurement Trust (Sykehusinnkjøp HF)
The Pharmaceutical strategy was passed by the board of The Norwegian Hospital Procurement Trust in the fall of 2018. This is the first category strategy of the 13 procurement categories for which The Norwegian Hospital Procurement Trust is responsible.
1. A Brief Outline of the Pharmaceutical Strategy
Good drug procurement ensures availability of drugs to the specialised health service and limits the cost increase.
Drug treatment is vital to modern healthcare and contributes to substantial health benefits. At the same time, drug costs increasingly weigh on the budgets of the specialised health service. Health trusts spent NOK 8.7 billion on drugs in 2018. The expenditure for 2020 is expected to exceed NOK 10 billion.
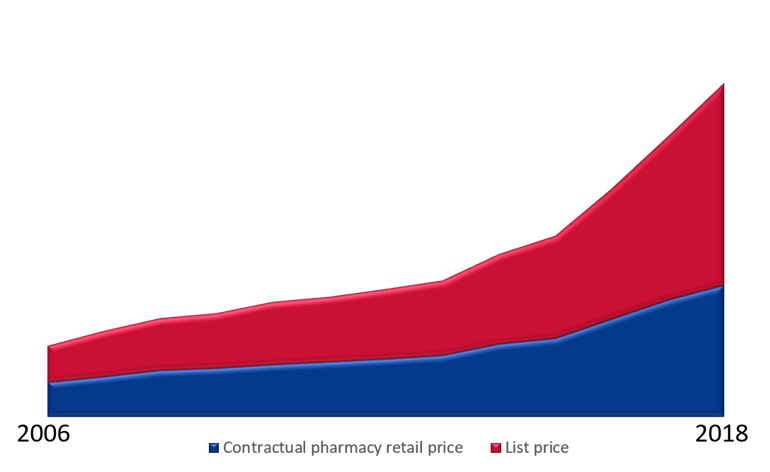
Figure 1: The figure above illustrates the development in pharmacy retail prices (list price) and contractual pharmacy retail prices (the retail price when the pharmaceutical is encompassed by an agreement made by the Sykehusinnkjøp HF). The difference between the pharmacy retail price and the contractual pharmacy retail price enables a growth in patient treatment in the specialised health service. Moreover, the figure shows how good procurements increasingly reduces drug expenditure and facilitates more extensive and better patient treatment.
Good pharmaceutical procurement can give the specialised health service quick access to effective pharmaceuticals at the lowest possible price, thereby facilitating more extensive pharmaceutical treatment or prioritisation of other important healthcare objectives.
Good procurements comprises both financial benefits and professional medical assessments, for example, in the areas of innovation, readiness and corporate social responsibility.
More extensive and better patient treatment through good pharmaceutical procurements.
Systems for good procurements have contributed to material benefits for the specialised health service for two decades. The Drug Strategy is based upon this. Procurement methods and practices will be further developed and additionally professionalised to fulfil the goal.
Goal achievement for The Norwegian Hospital Procurement Trust (hereafter refered by the Norwegian name Sykehusinnkjøp HF) requires resources to be used wherever they contribute to the goal to the greatest extent possible. Sykehusinnkjøp HF will be Norway’s leading procurement agency.
The main objective of pharmaceutical procurements will be realised through three focus areas, which will be followed up on by means of annual action plans:
Procuring pharmacetuicals in new and efficient ways:
What will be implemented?
Adapt procurements to the different life cycle stages of the pharmaceuticals.
Use procurement models that are suited to the competitive situation in the market.
Why will it be implemented?
Differentiated procurements will contribute to equal and quick access to effective drugs, at the lowest possible price.
• Suppliers must find it commercially attractive to offer new drugs and solutions.
How will it be implemented?
By using varied procurement methods.
- By using a procurement process that is optimal in the case at hand.
Ensuring responsible procurements:
What will be implemented?
Conduct procurements in a socially responsible way.
Ensure ethical trade, conduct procurements with environmental requirements and reward suppliers with sustainable production.
Why will it be implemented?
To ensure good quality in all stages, starting with the development and production of pharmaceuticals and ending with the prescription and use of pharmaceuticals.
The requirements regarding corporate social responsibility must contribute to realising significant social benefits.
How will it be implemented?
By basing the procurement requirements on the UN’s sustainable development goals and objective and measurable criteria.
By developing good and realistic requirements and implementing them in cooperation with the HTs, the market and the relevant authorities.
Effective implementation of procurements:
What will be implemented?
Facilitate good cooperation between the different participants to ensure compliance with the agreements.
Provide the management of the HTs and the Regional Health Authorities (hereafter referred to as RHAs) with information and support.
Why will it be implemented?
More effective implementation of procured agreements will contribute to equal and quick access to effective drugs.
New competence with regard to the procurement processes will contribute to the use of differentiated procurement models. This can also result in greater compliance with the agreements, which again will result in substantial savings.
How will it be implemented?
By clarifying the interaction between the National System for Managed Introduction of New Health Technologies (hereafter named New Methods) and the procurement process.
By following up on the implementation of and compliance with the agreements in cooperation with the management of the RHAs and HTs.
2. Background
This chapter contains the history behind the Pharmaceutical Strategy.
The Pharmaceutical Procurement Cooperation (hereafter referred to with the Norwegian abbreviation LIS) has been responsible for procuring pharmaceuticals for the specialised health service since 1995. LIS was transferred to Procurement services for Health Enterprises Ltd (Helseforetakenes innkjøpsservice AS, hereafter referred to with the Norwegian abbreviation HINAS) in June 2015. The Norwegian Hospital Procurement Trust (Sykehusinnkjøp HF) was then established in December 2015. On 1 November 2016, HINAS and LIS were transferred to Sykehusinnkjøp HF. During the transfer of operations, LIS was assigned to the Pharmaceutical Division.
The Pharmaceutical Division is one of six divisions in Sykehusinnkjøp HF and is entrusted with the following main tasks:
The responsibility for procurement of pharmaceuticals for the specialised health service.
Managing master data in the settlement scheme.
Conducting price negotiations for new drugs and participating in New Methods.
An analysis of the pharmaceutical division was presented at the Board meeting on 16 June 2017 (ref. no. 058-2017). The analysis provided an insight into the category, its complexity as well as special challenges. The Board passed a decision that the Chief Executive Officer should set up a working schedule for the strategy work with the category.
The work with the Pharmaceutical Strategy was launched in November 2017. The Strategy would build on the Sykehusinnkjøp HT's vision, objectives and values and on the methodology for preparing a category strategy drawn up in the “National Coordination and Standardisation” project (referred to in Norwegian with the abbreviation NSSIL). The goal is a strategic direction and an ambition that keeps with mission and governing documents from the RHAs and political documents from the Norwegian Ministry of Health and Care Services (hereafter referred to with the Norwegian abbreviation HOD).
The Strategy conforms with goals set out in reports from the Norwegian Parliament, political documents from HOD and mission and governing documents from the RHAs. This is linked to all levels within the management hierarchy.
Several reports have analysed the procurement of pharmaceuticals and the development in pharmaceutical expenditure in the specialised health service. There are, in particular, three factors that underpin the need for a pharmaceutical strategy:
Pharmaceuticals stand for an ever increasing share of the budgets of the specialised health service - The financing responsibility for more costly medicines is being transferred from the national insurance scheme to the specialised health service. This will be reflected, to an ever increasing extent, in the budgets of the health trusts until the transfer is complete in 2022.
The cost increase in the pharmaceutical area will be driven by greater application of new pharmaceuticals and therapeutic principles. OECD points out that the use of medicines increasingly shifts towards highly priced specialised pharmaceuticals. In addition, the starting price for pharmaceuticals in certain therapeutic areas are higher than ever.
A constantly evolving competitive environment - The position of a pharmaceutical in the life cycle effects the market situation and the development of it. Knowledge and competence must be developed based upon this.
The purpose of the Pharmaceutical Strategy is to live up to the assignments and orders given by the RHTs to Sykehusinnkjøp HF for pharmaceuticals. As a result, the Sykehusinnkjøp HF must act in a coordinated and coherent manner, in cooperation with the specialised health services. This will help to create good, fair and equitably distributed healthcare within the framework of the resources which are available to the health trusts at any given time.
To achieve the purpose, the strategy must balance several factors. The supplier market affects the ability of the specialised health service to deliver according to its objectives. The Pharmaceutical Strategy aims to set the tone for a good business dialogue between the specialised healthcare service and the pharmaceutical industry.
The strategic direction in the strategy process was set through contributions from a wide group of stakeholders. This was done through roundtable discussions, input rounds and interviews.
The dialogue with the stakeholders has been important when balancing the concerns of all parties in the pharmaceuticals’ value chain. The various perspectives have provided a good basis for identifying:
which areas that should be given a priority,
which areas that contain conflicting goals,
the areas which require more information.
All the input received has been actively used in the strategy process. Participation from the users will be vital to the future work and operationalisation of the strategy.
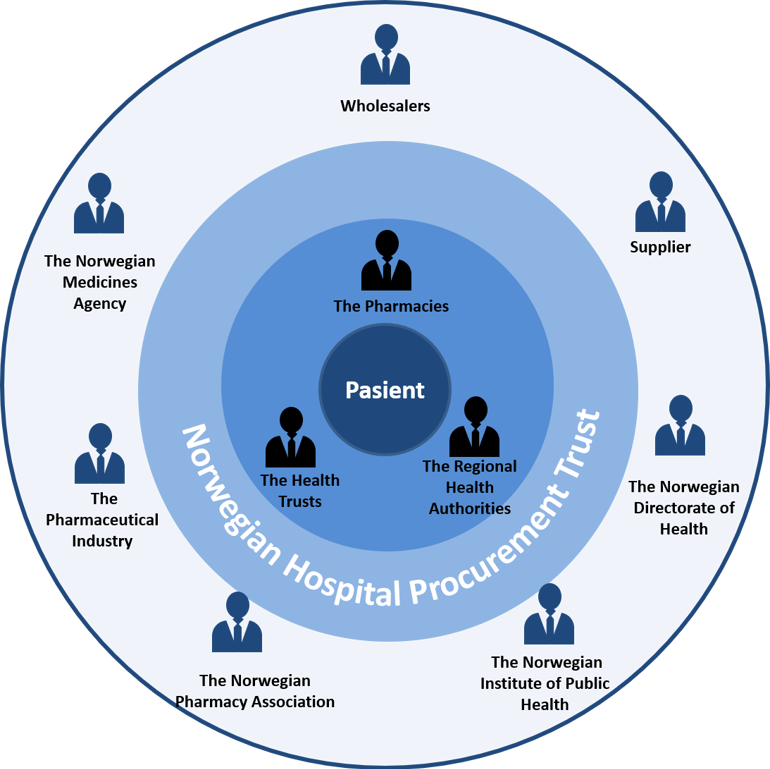
Figure 3: Overview of the stakeholders and parties in the specialised health service.
3. A Brief Outline of the Present-Day Situation
OECD points out two main concerns for the cost development in the pharmaceutical market; the use of more specialised pharmaceuticals, and increasingly high starting price in individual therapeutic areas. The analyses of pharmacetuical expenditure in the specialised health service indicate that both these effects are extremely relevant for Norway:
There are 50 pharmaceuticals (1.5 % of all drugs), which stand for 70 % of the pharmaceutical costs in the specialised health service in 2016. The majority of these are specialised pharmaceuticals with limited competition and often a high unit price.
The starting price for pharmaceuticals in Norway is normally based on the average of the three lowest prices in selected European countries. This means that Norway is affected by the same high starting price in individual therapeutic areas as the other OECD countries. This is also observed in the statistics of the Norwegian Pharmacy Association, which indicates that the average maximum price for the 20 most expensive drug packages has increased with 260 % from 2010 to 2018.
The pharmaceutical area is facing the same challenge as the rest of the healthcare industry. There is a growing discrepancy between what is medically possible and the resources that are available. This is referred to as a steadily growing “healthcare gap”. Pharmaceuticals are key to the modern healthcare, and proper use of pharmaceuticals can generate substantial health benefits for patients. However, many pharmaceuticals are costly, and the introduction of new ones can be demanding within the framework of the resources available to the health service.
The illustration below shows the development in the pharmacy retail prices and contractual pharmacy retail prices (where a discounted contractual pharmacy retail price) makes a growth in patient treatment activities possible. Moreover, the figure illustrates how good procurements increasingly reduces pharmaceutical expenditure and facilitates more extensive and better patient treatment.

Figure 4: An illustration of the cost development between the pharmacy retail price and the contractual pharmacy retail price in the period from 2010 to 2018.
Good procurement of pharmaceuticals is one of the most effective instruments the specialised health service has at its disposal to safeguard the availability of pharmaceuticals. It also limits the cost increase. In 2018, it contributed to making available resources corresponding to NOK 4.7 billion. This is a reduction of 35 % compared to the maximum pharmacy retail price (max. AUP). A more consistent transition to pharmaceuticals which are more reasonably priced and technically equivalent has a savings potential of additional NOK 1 billion.
Through several years of structured work, Sykehusinnkjøp HF has built an effective system for cooperation with professionals in the specialised health service and suppliers. Elements that are highlighted include LIS specialist groups, LIS contacts, LIS specialist seminars and dialogue meetings with the pharmaceutical industry.
Additional venues to exchange experience and facilitate discussions should be established between the specialist health service and the pharmaceutical industry. This presupposes that the dialogue has an actual impact on how procurements are carried out. A good dialogue regarding the process in New Methods and the procurement process will contribute to equal and quick access to effective drugs.
A consumption analysis has revealed a significant untapped potential resulting from a lack of compliance with the agreements. Two of the reasons being insufficient information and because the prescribers lack up-to-date information regarding the agreements. Electronic systems for prescription support can ensure that the benefits are realised through good implementation and compliance with agreements and recommendations.
4. The Pharmaceutical Strategy
The Pharmaceutical Division will deliver in accordance with the goal for the establishment of the Sykehusinnkjøp HF:
“The Sykehusinnkjøp HF shall provide a specialised and professional procurement service to the specialised health service”
This requires an integrated pharmaceutical and professional financial model, where cross-disciplinary cooperation with clinical professional circles is given pride of place. The development requires predictable and efficient processes, clarification of roles, responsibilities and cooperation with the different parties in the value chain.
The Pharmaceutical Strategy of the Sykehusinnkjøp HF underpins its goals. This is why the following main goal has been formulated for the strategy period:
“More extensive and better patient treatment
through good procurement of pharmaceuticals”
The main goal will be realised through three focus areas in the upcoming strategy period:
- Procuring pharmaceuticals in new and efficient ways
- Ensuring responsible procurements
- Implementing procurements more efficiently
The focus areas will help the Pharmaceutical Division to manage the responsibility for pharmacetuical procurements according to the needs of the specialised health service. Action plans will be prepared for each focus area and will be followed up on annually through the Pharmaceutical Division’s business plan. The achievement of goals will be assessed based on parameters set up for each focus area.
5. Priority Focus Areas
The focus areas are prioritised on the basis of 19 hypotheses. Prioritisation is based upon the following criteria:
- The Owners’ expectations.
- Contribution to goal achievement.
- The ability to influence/feasibility.
- Feedback from the stakeholders.
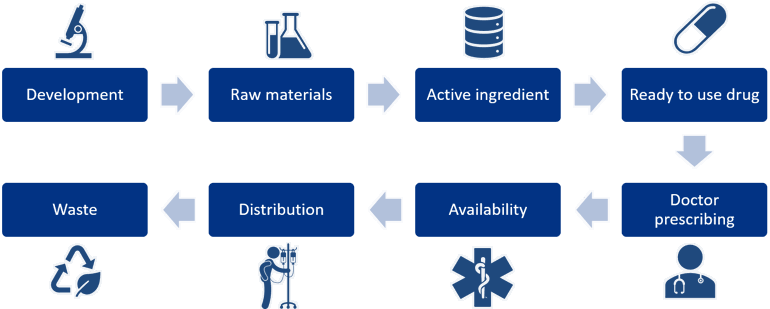
Figure 5: The complete value chain of a pharmaceutical.
Introduction
Procurements must be adapted to the market situation and the current phase in the pharmaceutical’s life cycle. The competitive situation in the different phases of a pharmaceutical’s life cycle is different. It is therefore necessary to employ different approaches when procuring. Different situations may call for a differentiated approach to confidentiality.
A differentiated approach should also make it predictable and commercially attractive for suppliers to offer new pharmaceuticals and methods.
The different phasess of a pharmaceutical’s life cycle
In order to understand the supplier market, an understanding of a pharmaceutical’s life cycle is important. A simplified version of a pharmaceutical’s life cycle and a definition of the different phases are presented in the figure below.
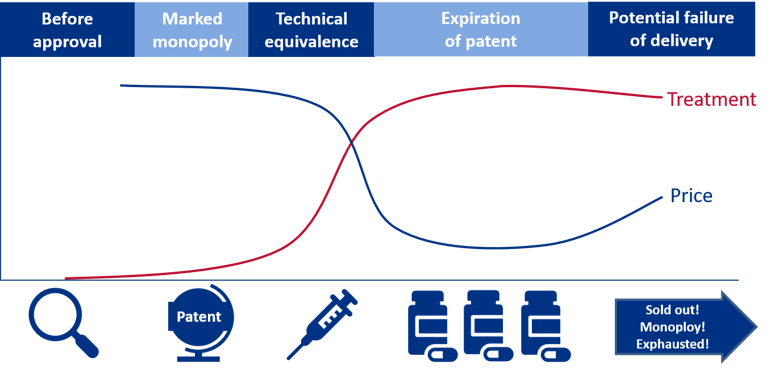
Figure 6: The figure illustrates two dimensions: the development in price per unit and the number of units sold. The graph is meant to illustrate a process where the unit price decreases over time, whereas the volume (e.g. number of packages) increases. All phases are vital when ensuring good patient care.
The various phases of a pharmaceutical’s life cycle:
- Phase 0 – Prior to having a marketing authorisation and registration exemption: The use of the pharmaceutical is essentially limited to clinical studies, “compassionate use programmes”, “named patient use” or approval exemptions.
- Phase 1 – Monopoly or extremely limited competition: In this phase, it may be possible to conduct price negotiations. There are no corresponding products on the market. The new pharmaceutical will be protected by patent.
- Phase 2 – Technically equivalent pharmaceuticals: The pharmaceutical is protected by patent during this phase, but one or more therapeutically equivalent pharmaceuticals are available on the market. This paves the way for competition between the technically equivalent pharmaceuticals.
- Phase 3 – Generic and bioequivalent competition: The patent on the pharmaceutical has expired. Suppliers with generic or bioequivalent active ingredient will enter the market. This creates a real competition between the pharmaceuticals.
- Phase 4 – Increasing price competition: More suppliers in the market and a substantial reduction in prices.
- Phase 5 – Intense price competition: The prices have stabilised at a low level. The number of suppliers is reduced due to a very intense price competition, where not all suppliers manage to offer competitive prices.
- Phase 6 – Pharmaceuticals with critical supply, no competition/monopoly: The development from stage 5 can go in several directions. For some pharmaceuticals, the competition remains intense, and the prices stabilises at a low level. For other pharmaceuticals, the price can become so low as to force suppliers to withdraw from the market. This leads to less competition, potentially higher prices as well as an increased risk of shortages.
A Differentiated approach to procuring
A differentiated approach to procuring implies an assessment of the market situation and life cycle of the individual pharmaceutical. The procurement strategy assesses which professional procurement instruments are most effective for the procurement in question. This may imply a novel approach.
The procurement packages in the figure below are placed in the procurement matrix where the y-axis stands for differentiation based on the professional medical complexity of a procurement, whereas the x-axis is based on market conditions. The figure illustrates how a pharmaceutical passes through the various phases of its life cycle.
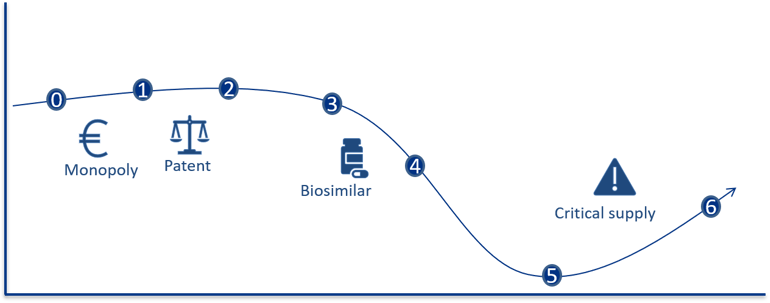
Figure 7: Procurement matrix throughout the pharmaceutical’s life cycle. Depending on the position in the procurement matrix, there will be different measures and assessments that must be undertaken in connection with the upcoming procurements.
The pharmaceutical’s life cycle and innovation
The Pharmaceutical Division will work actively with the areas where procurements affect the innovation created in the market. The Pharmaceutical Strategy must be considered against other instruments for innovation in the pharmaceutical area. The phase of the pharmaceutical’s life cycle influences which instruments that may be used. The Pharmaceutical Division of the Sykehusinnkjøp HF will primarily function as a knowledge base, making the health trusts and their employees aware of how existing pharmaceuticals can be used in new ways through professional procurements:
The innovative value of newly patented pharmaceutical will be assessed by New Methods and the Decision Forum. The Pharmaceutical Division will make a procurement/negotiation strategy based on guidelines from the Decision Forum.
The Pharmaceutical Division’s role in connection with innovation in phase 1 to 6 is to carry out procurements that encourages new methods to already known challenges. Procurements will be conducted in a way which contributes to:
- Balancing the procurement and professional medical aspects in order to achieve quick access to pharmaceuticals (phase 2).
- Strengthening the suppliers’ motivation to ensure stable and secure deliveries in order to thereby achieve better patient safety (phase 4 to 6).
New use of existing pharmaceuticals (phase 3 to 6): Methods that enables a greater value compared to comparable methods. This may apply to areas that provide increased patient safety, quality, reduced wastage, etc.
Introduction
There are growing expectations in both Norway and at an international level that public authorities and private companies safeguard corporate social responsibility in their own supply chains. The statutes of the Sykehusinnkjøp HF stipulate, as follows:
- The Health Trust shall ensure that procurements are organised in a proper and socially responsible way.
- The Health Trust shall be a driving force for ethical trade and environmentally-friendly procurements.
The UN’s member states have adopted 17 shared global goals for sustainable development by 2030. They are referred to as the UN’s sustainable development goals and consider the environment, economy and social development as a whole. Therefore, it is natural to use the UN’s sustainable development goals as a basis for the environmental and corporate social responsibility work when procuring pharmaceuticals.
In order to ensure good quality in pharmaceutical treatment, it is necessary to maintain good quality from the development of the pharmaceutical to prescription and application. It is furthermore important to be aware of environmental risks and the risk of human rights violations. Pharmaceuticals that end up in the nature’s cycle pose a substantial risk to the biological processes of various organisms. The risk of development of bacterial resistance is an important factor for antibiotics. When parts of or the entire production of a pharmaceutical take place in developing countries, it is particularly difficult to eliminate the risk of human rights violations or environmental concerns. An increased focus on the environment and corporate social responsibility will contribute to the achievement of goals within these areas.
Three focus areas when working with corporate social responsibility
The Sykehusinnkjøp HF will focus on three main areas of corporate social responsibility in the Pharmaceutical Strategy:
- Statutory framework: The requirements placed for approval of pharmaceuticals will have a direct effect on how requirements are implemented in the pharmaceutical’s value chain.
- Nordic cooperation: A cooperation amongst the Nordic countries increases the market impact. It is expedient for the pharmaceutical market that the Nordic countries place the same requirements for corporate social responsibility.
- The supplier market: A good cooperation with the participants in the market is a prerequisite for achieving good solutions. A common understanding of what is required sets the terms for differentiated procurements, including within the area of corporate social responsibility.
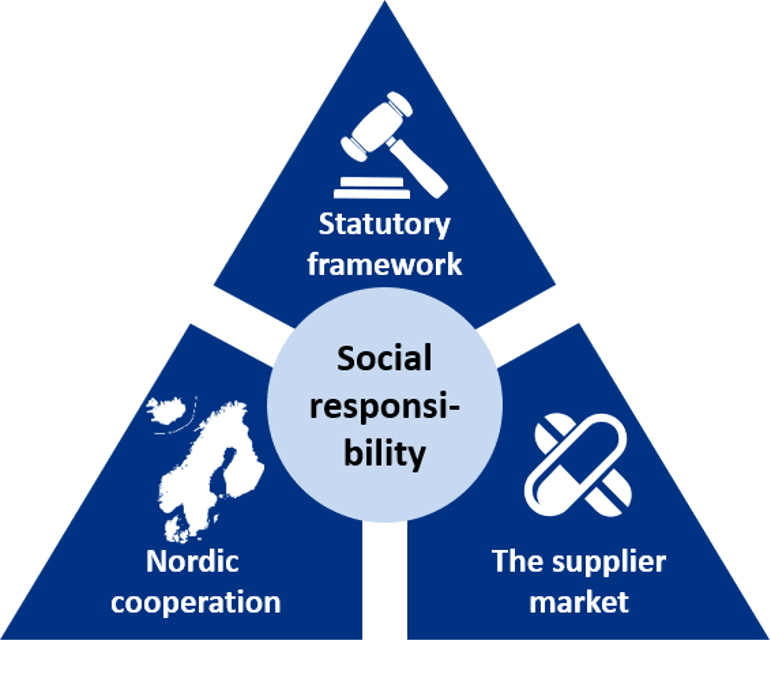
Figure 8: Working with corporate social responsibility.
Corporate social responsibility in the procurement process
The Pharmaceutical Strategy will be based on regulations on corporate social responsibility in public procurement. The Norwegian Public Procurement Act requires public enterprises to organise their procurement practices in such a way as to safeguard the environment, human rights and other social considerations. The Strategy will contribute to carrying out procurements that reduces adverse environmental impact and promotes climate-friendly solutions, wherever relevant.
The procurement processes will ensure safeguarding of the environment and climate, fundamental human rights and anti-corruption efforts. Corporate social responsibility must be viewed in the perspective of the value chain, where measures are assessed based on efficacy and feasibility.
The following principles will be used as a basis in the work with being a driving force for corporate social responsibility in public procurements:
- The requirements are based upon the UN’s sustainable development goals.
- Requirements for corporate social responsibility are drawn up in cooperation with the market.
- The preparation and follow-up of requirements are based upon objective and measurable criteria.
- Requirements are to be clarified and anchored with owners/budget managers before being used.
Introduction
The Sykehusinnkjøp HF will contribute to faster implementation, better compliance with agreements and faster access to pharmaceuticals. This will be achieved by creating a good flow of information between parties on the purchaser’s side. In addition, there will be created good venues for market dialogue and cooperation.
Pharmaceutical procurement agency
The Sykehusinnkjøp HF is a pharmaceutical procurement agency for the specialised health service. Sykehusinnkjøp HT will contribute to choosing good solutions, which align with the interests of society and political and medical guidelines in the pharmaceutical area.
The Strategy addresses the interface between procurement and pharmaceutical professionals. Pharmaceuticals are selected based on criteria that safeguard financial, supply, ethical and medical concerns. The role as a pharmaceutical procurement agency comprises the pharmaceutical’s entire value chain, from production to patient therapy.
Implementation of and compliance with agreements
The specialised health service fails to realise major gains when agreements are not complied with. Efficient compliance with signed agreements is vital to achieving the best possible results in future procurement processes.
Efficient compliance with the agreements calls for a good implementation process. Through the Pharmaceutical Strategy, the Sykehusinnkjøp HF takes an extended responsibility for providing good decision-making information in procurement and pharmaceutical-related issues to the management of the Health Trusts and the Regional Health Trusts.
Once a procurement has been conducted, and there is a result and recommendation, the following prerequisites must be in place:
- A close cooperation between the Pharmaceutical Division of the Sykehusinnkjøp HT and the management of the Health Trusts and the Regional Health Trusts.
- Information about the pharmaceuticals are made available and structured in order to support proper prescription and application of pharmaceuticals in the specialised health service.
- A close cooperation between the Pharmaceutical Division of the Sykehusinnkjøp HT and contract suppliers, wholesalers and pharmacies.
Interface between New Methods and the procurement process
The Sykehusinnkjøp HF’s task in New Methods is to carry out negotiations whenever a lower price is required in order to assess a pharmaceutical as cost-effective or when a cost-effective pharmaceutical has substantial budgetary consequences. The Sykehusinnkjøp HF will exercise its mandate to draw up therapeutic conditions for cost-effective administration. The Pharmaceutical Division of the Sykehusinnkjøp HF has also been given a mandate to enter into national agreements for the use of new pharmaceuticals without marketing authorisation in “compassionate use programmes” on behalf of the regional technical directors.